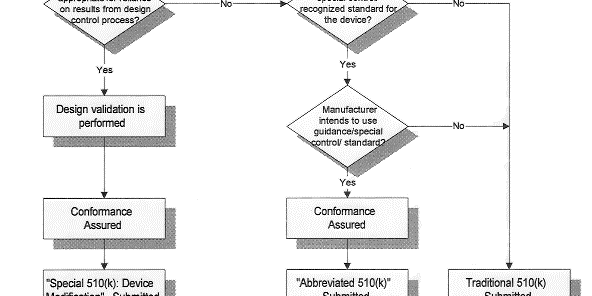
As a medical device consultant, PDG takes great interest in the ever-changing landscape of the premarket notification (510(k)) review and clearance process. Besides the significant scrutiny given to the program over the past five years, the vast majority of legally marketed medical devices in use in the U.S. today were cleared via the 510(k) regulatory pathway.[1] The statutory mechanism giving birth to the 510(k) (as well as the IDE and PMA) was the Medical Device Amendments to the Federal Food, Drug, and Cosmetic (FD&C) Act, enacted on May 28, 1976. This was the same law that established the Class I, II, and III, regulatory categories describing the degree of regulation FDA believes is necessary to reasonably assure the public of the safety and effectiveness of marketed medical devices.[2] [3] The 510(k) requires device manufacturers to notify FDA of their intent to market a medical device at least 90-days in advance of launch (review times vary). This allows FDA to determine whether the device is substantially equivalent to a predicate (a device already placed into one of the three classification categories). This facilitates de facto identification of devices not in commercial distribution prior to May 28, 1976 as “new” devices requiring clearance or approval.[4] [5] The 510(k) was originally intended to address all but Class III devices requiring a PMA. However, in 1997, FDAMA eliminated the need for a 510(k) filing for most Class I and some Class II devices (known as “exempt devices”). 510(k)s are generally required in three scenarios: introduction of a new device into the stream of commerce for the first time, intended use is different than that of the predicate already in commerce, or a legally marketed device changed or modified in a way that could significantly affect its safety or effectiveness.[6] Today, there are 4 types of 510(k) submissions. The table below describes each, as well the percentage each represented of total 510(k) submissions in 2012: [7] [8] [9]
The Four Types of 510(k) Submissions
[wpsm_comparison_table id=”17″ class=”customTable threeColumn”]
Over the past five years, the 510(k) process has been the subject of a great deal of public policy review. On Aug. 3, 2010, CDRH released a preliminary internal evaluation recommending changes to the 510(k) process.[10] Asked by FDA to review the process, by 2011 the Institute of Medicine (IOM) reported that “Stimulated by reports of problems with several 510(k)-cleared devices, the public, legislators, the Government Accountability Office, the Department of Health and Human Services Office of the Inspector General, and the courts, including the Supreme Court, have all questioned the logic and value of the 510(k) clearance process being used by a federal agency charged with responsibility for protecting and promoting the public’s health. After 35 years, at a time of rapidly changing science and technology, questions persist about whether the 510(k) process is protecting and promoting the public’s health.”[11] The emergence of such an outcry for change came as little surprise to anyone familiar with the industry. By 2013, FDA was refusing to accept 60% of 510(k) submissions. Many at the time believed the era of the 510(k) might be coming to an end. However, The 510(k) survived and in 2015 FDA issued a report noting that “Over the past five years, the Food and Drug Administration’s device program has shown a pattern of markedly improved performance. Today it is performing strongly across a wide range of performance measures. At the same time, FDA has implemented a range of initiatives to promote access to safe and effective medical devices for American patients.”[12] Indeed, between 2013 and August of 2015, CDRH issued 25 new draft or final guidances. Between Q1 2014 and Q1 2015, RTA rates fell 57% and had dropped to 37% by Q2 2015. In addition, timeframes for 510(k) reviews were reduced (as well as timeframes for PMAs, IDEs and de novo reviews). Cited, among other factors, were the “various internal policy and program changes since 2010”[13] Clearly, the quality of 510(k) submissions has improved. However, RTA rate is still high. In fact, the three most common reasons for the rejection of a 510(k) by CDRH are failure to adequately present biocompatibility and shelf-life data, failure to incorporate FDA feedback, and lack of full test reports.[14] Fortunately, such deficiencies are highly preventable. The U.S. medical device market is the largest in the world. With the proper planning and foresight, 510(k) clearance can pave the way to relatively rapid entry into many other lucrative markets throughout the world. However, this requires expert navigation of the complexities of the 510(k) process. Many manufacturers encounter setbacks in their development efforts, preventing prompt review by CDRH. The result is costly delays in clearance and launch of new devices. However, manufacturers can reduce the probability of such delays and incorporate a global strategy into U.S. development efforts by becoming more knowledgeable about FDA regulations and guidances, monitoring the ever-changing regulatory landscape, and working with a medical device consultant experienced in the preparation of 510(k) submissions. Charles Jaap is Vice-President of Operations and Business Development for PDGTM, a global pharmaceutical consultant with extensive experience in the strategic development of 505(b)(2) drug products. Please feel free to contact us for more information. The opinions and statements in this paper are solely those of Charles Jaap and do not necessarily reflect those of PDG.